WorkfloPlus for Pharmaceuticals & Life Sciences
Digital Work Instructions for GxP-Ready Operations
In pharmaceuticals and life sciences, getting the process right isn’t just about efficiency. It’s about compliance, data integrity and patient safety.
Procedures must be followed precisely. Evidence has to be captured correctly. And when an auditor asks for proof, you need to be able to show exactly what happened, when it happened and who did it.
WorkfloPlus by Intoware is built for that reality.
Instead of relying on paper forms, static PDFs or manual data entry, WorkfloPlus turns your approved SOPs into interactive digital work instructions. Operators are guided step-by-step, while ALCOA+ compliant evidence is captured automatically as the work is performed.
The result is simple: consistent execution, reliable documentation and audit-ready records – without creating extra admin.
Turning SOPs Into Executable Processes
Most pharmaceutical manufacturers already have well-written procedures. The challenge isn’t creating SOPs, it’s proving they were followed.
Traditional paper-based processes create problems. Documentation is completed after the event. Information gets missed or entered incorrectly. Evidence is scattered across forms, photos and spreadsheets. And when an audit arrives, preparing the records can take days or weeks.
WorkfloPlus removes the gap between procedure and proof.
Each procedure becomes a structured, guided process that ensures the right steps are followed in the right order. Mandatory fields, validations and in-process checks mean critical information can’t be skipped. Photos, measurements, digital signatures and timestamps are captured in the moment, creating a complete record of what actually happened.
Instead of asking operators to “fill in forms”, you give them a tool that helps them do the job correctly, and generates compliant documentation as a natural by-product.
Designed for Regulated Environments
WorkfloPlus has been developed with pharmaceutical and life sciences requirements in mind.
Every action within a procedure is attributable to a specific user. Records are created automatically at the point of execution. Data is structured, secure and fully traceable. Version control ensures that operators always work from the latest approved procedure.
For those working to FDA 21 CFR Part 11, Annex 11 or broader GxP standards, this provides a practical way to digitise frontline processes while maintaining control and data integrity.
WorkfloPlus provides quality and operations teams with a platform that understands how regulated work actually gets done.
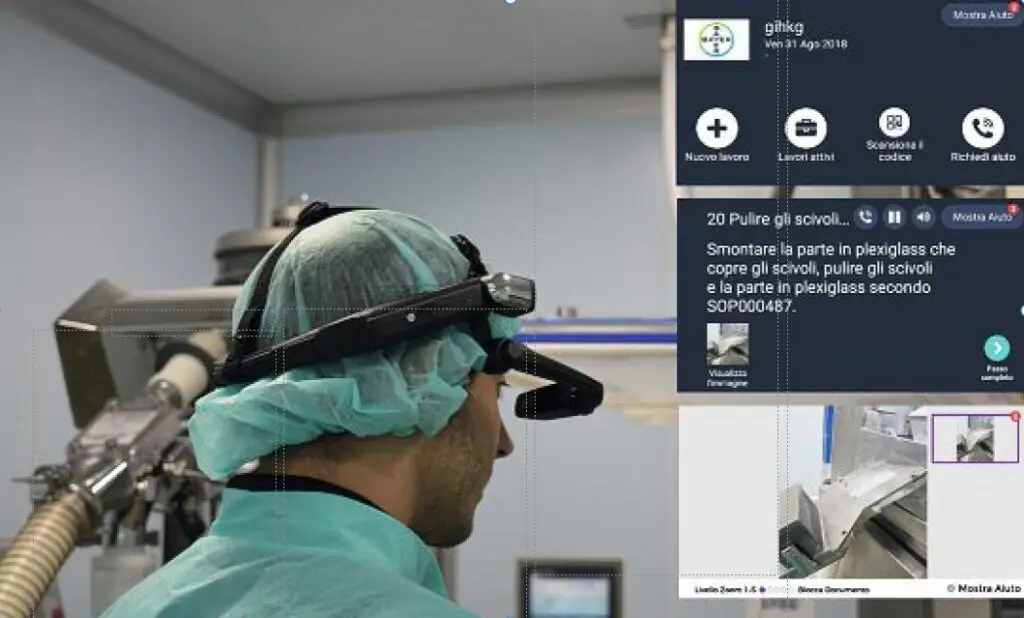
Real-World Results: Bayer Case Study
The value of this approach is already proven in pharmaceutical operations.
At Bayer’s Garbagnate facility in Italy, WorkfloPlus was introduced as part of a wider Industry 4.0 initiative to improve changeover processes and operational transparency.
By replacing paper-based instructions with augmented digital workflows, the team were able to standardise complex activities, capture structured execution data in real time and significantly reduce changeover times. More importantly, they gained visibility into how work was really being performed on the shop floor.
The project showed how digital work instructions can deliver two things at once: stronger compliance and better productivity.
Built for Every Stage of Pharmaceutical Operations
Process Execution

Get complex procedures right first time
WorkfloPlus turns approved SOPs into guided, step-by-step digital workflows that operators follow in real time. Each instruction is validated, each action is confirmed, and every critical step is captured as it happens. This removes ambiguity, reduces human error and ensures procedures are performed exactly as intended – every shift, every batch, every time.Evidence Capture
Get proof in the moment it happens
Photos, measurements, digital signatures and operator confirmations are captured directly within the process. Everything is time-stamped, attributable and stored securely in a single digital record. No retrospective paperwork. No missing details. Just complete, ALCOA+ compliant documentation created naturally as part of doing the job.Audit-Ready Traceability

Total visibility across people, processes and data
WorkfloPlus links every action, check and result into a structured audit trail. You can see who performed a task, which version of a procedure was used, and exactly what data was recorded. If an issue arises, the full history is available instantly – without chasing forms, spreadsheets or paper folders.GxP Compliance by Design

Make compliance part of the process, not an admin task
With WorkfloPlus, compliance isn’t something added at the end. It’s built into everyday activity. Mandatory fields, validations and controlled processes ensure Good Documentation Practice is followed automatically, creating reliable records for FDA, MHRA, EMA and internal quality standards.Knowledge Capture and Standardisation

Protect critical expertise
Experienced operators know how to perform tasks correctly. WorkfloPlus captures that know-how digitally and embeds it into repeatable procedures. Best practice becomes standard practice, even as teams change and new staff join.Quality Assurance and Inspections

Prove quality with real evidence
From in-process checks to line clearance and cleaning verification, WorkfloPlus ensures that inspections are performed consistently and documented correctly. Visual evidence and structured data replace handwritten notes, making internal and external audits faster and more reliable.Training and Competency

Train operators in the flow of real work
New starters can learn directly from following validated guided procedures while capturing real evidence. This reduces onboarding time, improves consistency and gives quality teams confidence that tasks are being performed by competent, supported staff.Operational Insight
Capture data other systems miss.
WorkfloPlus collects structured execution data directly from the people doing the work. You gain visibility into how long processes really take, where deviations occur and where improvements can be made – turning everyday activity into actionable intelligence.Trusted by












Our Approach
Intoware’s customer success model ensures your project achieves it’s goals, and provides ROI as quickly as possible.
Engage
On our Discovery call, we’ll find out about you and your existing processes to learn exactly what you’re looking for.
Design
Configure your workflows. Customise templates with our toolkit or build processes from scratch.
Deploy
Integrate your digital workflows with existing devices. We’re on hand to help with training, support and integration.